

#Molecular geometry series
The mistake caused more debate online on the topic of Biden’s mental state, coming hot on the heels of a series of absent-minded blunders in recent weeks, including putting a medal on backwards and falling off his bike. ‘The percentage of women who register to vote and cast a ballot is consistently higher than the percentage of the men who do so…End of Quote. The president was announcing new policy in response to the Supreme Court’s decision to overturn abortion rights case Roe v Wade when he also read the teleprompter instructions on what to say.įlanked on one side by Vice President Kamala Harris, Biden was reading the speech when he finished the sentence with the instructions:
Moore, John W., Stanitski, Conrad L., Wood, James L., Kotz, John C., The Chemical World, Concepts and Applications, Second Edition, Volume 1, Harcourt Brace & Co., 1998, pages 397-447.US President Joe Biden delivers another concerning gaff while announcing ‘reproductive rights action’ on national television. Ebbing, Darrell D., Wrighton, Mark S., General Chemistry, Second Edition, Houghton Mifflin Co., 1987 pages 286-321.Ģ. To predict the molecular geometry select from the table below the 3D arrangement that has the same number of bond domainsand lone pairs of electrons.ġ. Step 4: The molecular geometry describes the position only of atomic nuclei (not lone electron pairs) of a molecule (or ion). Determine the number of bond domains and the number of lone pairs of electrons.ģ. Halogens and noble gases can expand their octet.Ģ.
#Molecular geometry how to
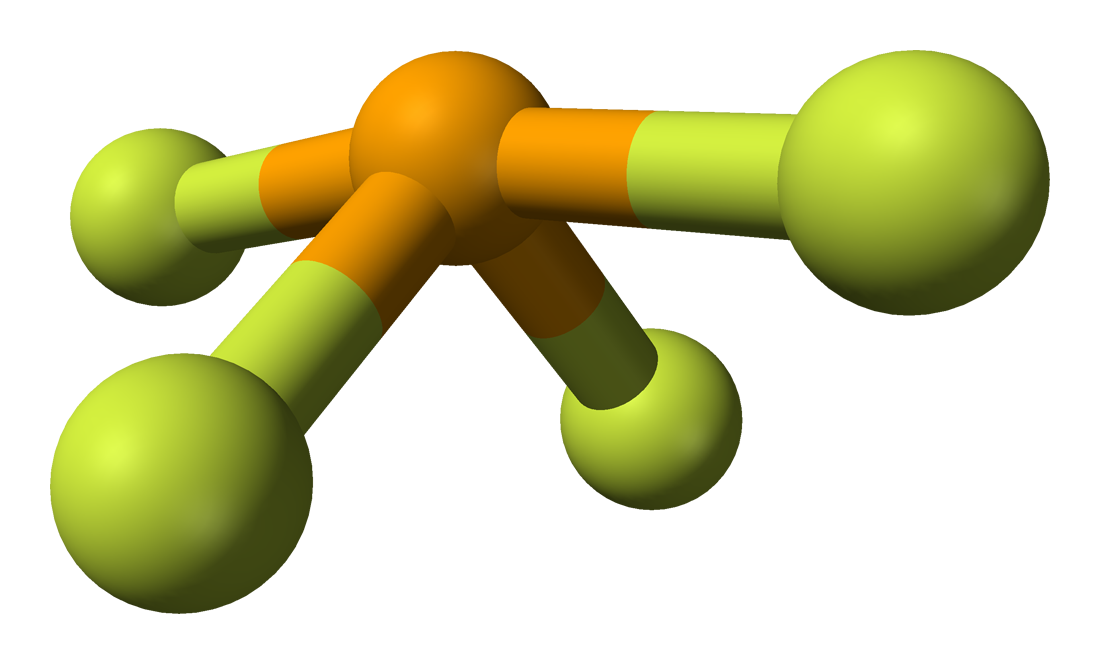
Note: To chose the central atom as the one with the smallest number of valence electrons or if they all have the same number of valence electrons then choose the one in the least amount. How to determine molecular geometry (molecular shape) using the table below: Determine the number of valence electrons for all the atoms in a molecule. Draw Lewis structures for the molecular formula given. How to use the table to predict molecular geometry.ġ. For example, the molecule ethylene, H 2C=CH 2, has the carbon-carbon atoms sharing four electrons but one bond domain between the two carbon atoms. In any case, if two atoms share two electrons or more they will have one bond domain. For ease of understanding we will call a bond domain the electrons that hold together two atoms. The following table will help you understand how molecular geometry can be predicted using the VSPER model. A molecule's shape can affect the physical.
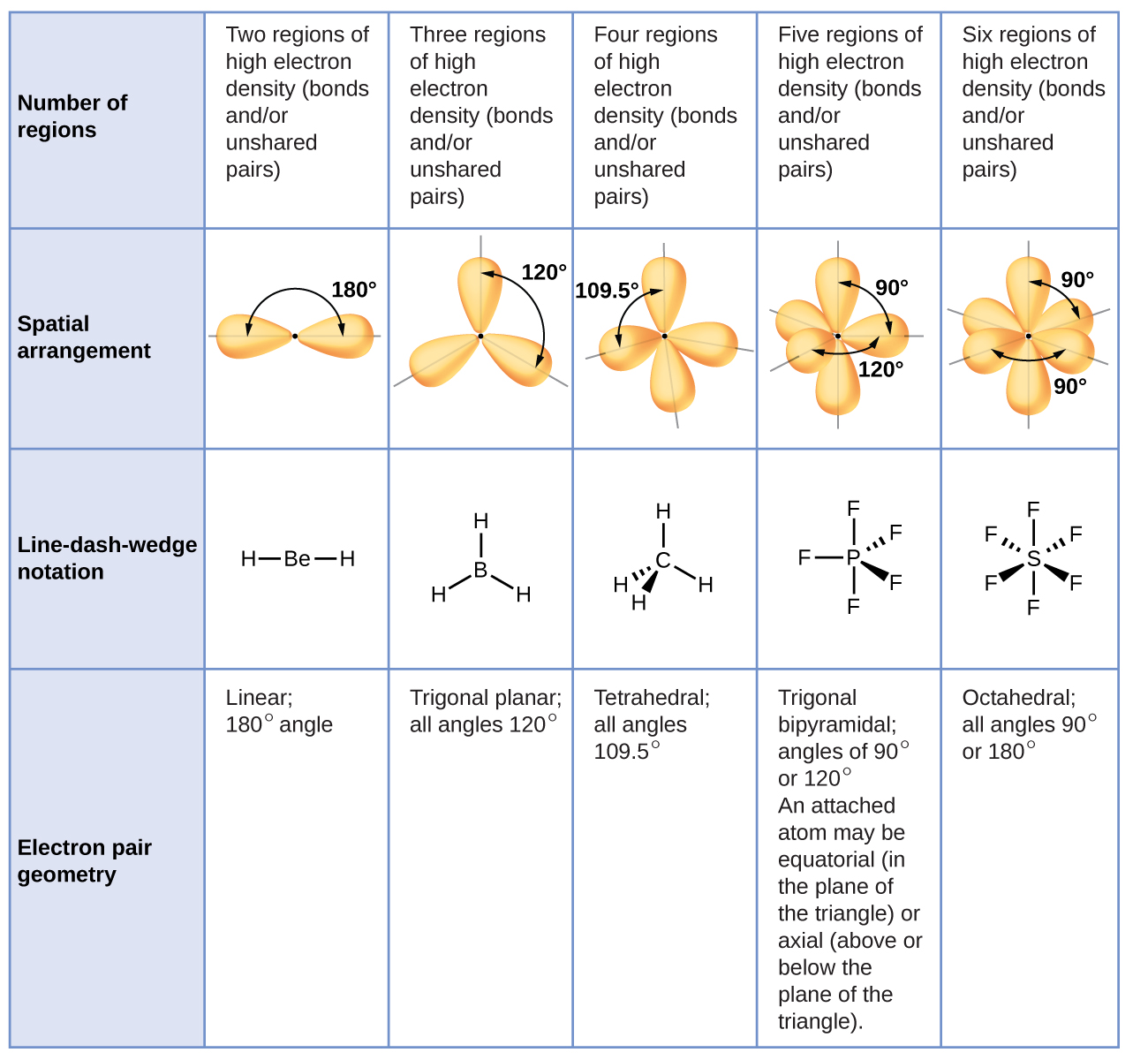
On the other hand it takes into account the very important Pauli exclusion principle where each electron pair must occupy a different spatial region about an atom. Molecular geometry studies the three-dimensional shapes molecules form and how these shapes relate to chemical reactivity and physical characteristics. On the first hand it minimizes repulsion between electrons due to electrostatic interactions. The VSPER model is based on two important principles. This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible. The valence shell electron pair repulsion (VSPER pronounced "vesper") model provides some useful tools for predicting molecular geometries. Atoms have a definite three-dimensional spacearrangement relative to each other in a molecule. can also be observed in the microscopic world. The geometrical arrangements seen in nature, i.e., flowers,stones, trees, etc.


 0 kommentar(er)
0 kommentar(er)
